The aim of this NINDS funded trial is to assess whether high dose Epo will improve survival without neurodevelopmental impairment (NDI) in infants born between 24 and 28 weeks of gestation (term is 40 weeks). 940 infants will be enrolled at 16 centers across the U.S. Subjects will be assessed at 2 years of age to see whether Epo treatment will improve neurodevelopmental outcomes.
Principal Investigators, Clinical Coordinating Center: Sandra Juul, MD, PhD
Principal Investigator, Data Coordinating Center: Patrick Heagerty, PhD
Funding: NINDS 1U01NS077953-01
ClinicalTrials.gov Identifier: NCT01378273
Prematurity is defined as a birth that occurs before 37 completed weeks of gestation. It is associated with about one-third of all infant deaths in the United States and accounts for approximately 45 percent of children with cerebral palsy, 35 percent of children with vision impairment, and 25 percent of children with cognitive or hearing impairment. (Ref: UpToDate).
The risk of complications of prematurity increases with increasing immaturity. Thus, infants who are extremely premature, born at or before 28 weeks of gestation, have the highest mortality rate, and if they survive, are at the greatest risk for long-term problems.
In preterm survivors, there is a high rate of long-term neurodevelopment impairment and chronic health problems. These chronic medical and neurodevelopmental complications often require additional health care and educational services, which add to the overall economic cost of caring for the premature infant.
Prematurity can be defined by gestational age or birth weight:
Approximately 50,000 infants per year (961 per week) are born at less than 28 weeks of gestation in the US. Cerebral palsy, deafness, blindness, and/or mental retardation are present in up to 50% of surviving extremely preterm infants at school age.
Perinatal care costs for these infants exceed US$18 billion every year. The burden of extreme prematurity to each patient and to society is further magnified by the years of productive life lost. New therapies are needed to improve these outcomes.
Erythropoietin Neuroprotection: The neuroprotective and neuroregenerative effects of high dose Epo have been well documented in experimental models of neonatal brain injury.1-7 While Epo neuroprotection for preterm infants holds translational promise, only 2 phase I/II trials have been published to date.8, 9 We predict the anti-apoptotic, anti-inflammatory,10-12 oligodendrocyte protective12-19 and neurogenic effects20-26 will decrease acute and chronic brain injury, and support normal brain development, thereby improving the neurologic outcomes of ELGANs. Pragmatically, Epo is FDA approved, widely available, affordable, and has proven safe in over 25 years of randomized controlled trials evaluating neonatal erythropoiesis.
Epo Neuroprotection. The hematopoietic cytokine Epo has neuroprotective and neuroregenerative effects in the brain.7 Mechanisms of Epo neuroprotection include receptor-mediated, cell specific effects that occur both early and late in the healing process, and non-specific effects that also modulate the response to injury. Epo has anti-inflammatory, anti-excitotoxic,27 anti-oxidant,28 and anti-apoptotic effects on neurons and oligodendrocytes, and promotes neurogenesis and angiogenesis, which are essential for injury repair and normal neurodevelopment. Epo effects are dose-dependent, and multiple doses are more effective than single doses.1, 21, 26 Epo reduces neuronal loss and learning impairment following brain injury,29, 30 and even when initiated as late as 48-72 hours after injury, there is evidence of improved behavioral outcomes, enhanced neurogenesis, increased axonal sprouting, and reduced WMI.16, 31
White matter injury. WMI is a common brain injury affecting preterm infants.32-34 Epo decreases WMI in adult and neonatal animal models of brain injury.12-14, 16, 35-38 Mechanisms for this may be Epo protection of vulnerable preoligodendrocytes: functional Epo receptors are expressed by immature oligodendrocytes, Epo promotes the proliferation, maturation and differentiation these cells,19 and protects them from injury induced by interferon-γ, lipopolysaccharide (LPS), and hypoxic-ischemia.16-18
Inflammation. Perinatal inflammation (chorioamnionitis, necrotizing enterocolitis (NEC), or sepsis) is associated with increased risk of NDI.39-41 Microglial activation and increased cytokine expression, particularly TNF-a, interleukin (IL)-6, and IL-8, have been associated with brain injury in preterm infants and in animal models of neonatal brain injury.42 Epo has demonstrated anti-inflammatory effects, which may contribute to neuroprotection in the scenario of preterm birth and increased inflammatory activity.10, 11, 43-47
Apoptosis. Neurons in the developing brain are more likely than adult neurons to undergo apoptosis if exposed to injurious stimuli,48, 49 and the anti-apoptotic properties of Epo may protect these vulnerable neurons.1, 22, 50
Repair. Epo stimulates growth factors required for normal brain growth such as brain-derived neurotrophic factor (BDNF) and glial cell derived neurotrophic factor (GDNF).20, 51 Epo enhances neurogenesis,20-26 angiogenesis, repair and plasticity, thus providing long lasting neuroprotective and trophic effects.21, 23, 31, 52-55
Reactive Iron. Iron is highly reactive and normally sequestered by transport proteins. Unbound iron produces free radicals and subsequent oxidative injury. Preterm infants have measurable free iron, which increases after transfusions of red blood cells or during metabolic instability such as sepsis.56-59 In our phase I/II study of Epo administration in extremely low birth weight infants we observed a transient increase in reticulocytosis, indicating an increase in iron utilization.60 Epo may contribute to neuroprotection by decreasing free iron.
Molecular mechanisms of Epo neuroprotection. EpoR are present on neuron progenitor cells,20 neurons,61 astrocytes,19 oligodendrocytes,62 microglia,63 endothelial cells20 and erythrocyte progenitors. Epo has direct neuroprotective effects via EpoR binding: Epo-bound receptors dimerize to activate anti-apoptotic pathways via phosphorylation of JAK2, phosphorylation and activation of MAPK, ERK1/2, as well as the PI3K/Akt pathway and STAT5, which are critical in cell survival.50, 63 Epo also functions through indirect effects, increasing iron utilization by increasing erythropoiesis, and by decreasing inflammation10, 11 and oxidative injury.64, 65
1. Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61(4):451-5.
2. Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006;317(1):109-16.
3. Yis U, Kurul SH, Kumral A, Tugyan K, Cilaker S, Yilmaz O, Genc S, Genc K. Effect of erythropoietin on oxygen-induced brain injury in the newborn rat. Neurosci Lett. 2008;448(3):245-9.
4. Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71(5):659-69.
5. Spandou E, Soubasi V, Papoutsopoulou S, Karkavelas G, Simeonidou C, Kaiki-Astara A, Guiba-Tziampiri O. Erythropoietin prevents hypoxia/ischemia-induced DNA fragmentation in an experimental model of perinatal asphyxia. Neurosci Lett. 2004;366(1):24-8.
6. Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67(6):795-803.
7. van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008;59(1):22-33.
8. Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122(2):383-91.
9. Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M, Bucher HU. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122(2):375-82.
10. Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36(8):1672-8.
11. Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485-92.
12. Rees S, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn M, Greenwood D, Harding R. Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J Neuropathol Exp Neurol. 2010;69(3):306-19.
13. Yamada M, Burke C, Colditz P, Johnson DW, Gobe GC. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. J Pathol. 2011.
14. Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS ONE. 2010;5(6):e11016. PMCID: 2884017.
15. Kim YJ, Jung YW. Systemic injection of recombinant human erythropoietin after focal cerebral ischemia enhances oligodendroglial and endothelial progenitor cells in rat brain. Anat Cell Biol. 2010;43(2):140-9. PMCID: 2998786.
16. Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41(5):1032-7. PMCID: 2919308.
17. Mizuno K, Hida H, Masuda T, Nishino H, Togari H. Pretreatment with low doses of erythropoietin ameliorates brain damage in periventricular leukomalacia by targeting late oligodendrocyte progenitors: a rat model. Neonatology. 2008;94(4):255-66.
18. Genc K, Genc S, Baskin H, Semin I. Erythropoietin decreases cytotoxicity and nitric oxide formation induced by inflammatory stimuli in rat oligodendrocytes. Physiol Res. 2006;55(1):33-8.
19. Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391-403.
20. Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732-7.
21. Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321-30.
22. Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011.
23. Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke 2007;38:2795-803.
24. Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733-43.
25. Osredkar D, Sall JW, Bickler PE, Ferriero DM. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis. 2010;38(2):259-65. PMCID: 2854222.
26. Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31(5):403-11.
27. Zacharias R, Schmidt M, Kny J, Sifringer M, Bercker S, Bittigau P, Buhrer C, Felderhoff-Muser U, Kerner T. Dose-dependent effects of erythropoietin in propofol anesthetized neonatal rats. Brain Res. 2010;1343:14-9.
28. Kumral A, Gonenc S, Acikgoz O, Sonmez A, Genc K, Yilmaz O, Gokmen N, Duman N, Ozkan H. Erythropoietin increases glutathione peroxidase enzyme activity and decreases lipid peroxidation levels in hypoxic-ischemic brain injury in neonatal rats. Biol Neonate. 2005;87(1):15-8.
29. Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58(2):297-301.
30. McPherson RJ, Demers EJ, Juul SE. Safety of high-dose recombinant erythropoietin in a neonatal rat model. Neonatology. 2007;91(1):36-43.
31. Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, Pluchino S, Gassmann M, Hermann DM. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134(Pt 1):84-99.
32. Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28(6):405-14.
33. Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr., Murdoch GH, Montine TJ. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58(1):108-20.
34. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110-24. PMCID: 2707149.
35. Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Fontana E, Gorio A, De Biasi S. Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience. 2008;151(2):452-66.
36. Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Kapke A, Lu M, Ewing JR, Chopp M. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40(3):936-41. PMCID: 2730918.
37. Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S, Bigini P, Barbera S, Fumagalli E, Mennini T, Vezzani A, Rizzi M, Coleman T, Cerami A, Brines M, Ghezzi P, Bianchi R. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172(1-2):27-37.
38. Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Gorio A, De Biasi S. Erythropoietin-mediated preservation of the white matter in rat spinal cord injury. Neuroscience. 2007;144(3):865-77.
39. Leviton A, Fichorova R, Yamamoto Y, Allred EN, Dammann O, Hecht J, Kuban K, McElrath T, O'Shea TM, Paneth N. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine. 2011;53(1):66-73. PMCID: 2987520.
40. Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, Leviton A. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr. 2010;157(5):751-6 e1. PMCID: 2952050.
41. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696-703.
42. Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J, Sizonenko S, Hagberg H, Lelievre V, Gressens P. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70(4):550-65.
43. Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. PNAS. 2002;99(14):9450-5. PMCID: 123161.
44. Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971-5. PMCID: 2194205.
45. Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. PNAS. 2001;98(7):4044-9.
46. Bian XX, Yuan XS, Qi CP. Effect of recombinant human erythropoietin on serum S100B protein and interleukin-6 levels after traumatic brain injury in the rat. Neurol Med Chir (Tokyo). 2010;50(5):361-6.
47. Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19(12):1701-3.
48. Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453-501.
49. McDonald JW, Behrens MI, Chung C, Bhattacharyya T, Choi DW. Susceptibility to apoptosis is enhanced in immature cortical neurons. Brain Res. 1997;759(2):228-32.
50. Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641-7.
51. Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177-87.
52. Ransome MI, Turnley AM. Systemically delivered Erythropoietin transiently enhances adult hippocampal neurogenesis. J Neurochem. 2007;102(6):1953-65.
53. Bocker-Meffert S, Rosenstiel P, Rohl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43(6):2021-6.
54. Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61(3):199-208.
55. Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, Feng Y, Zhang ZG. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28(7):1361-8.
56. Marzocchi B, Perrone S, Paffetti P, Magi B, Bini L, Tani C, Longini M, Buonocore G. Nonprotein-bound iron and plasma protein oxidative stress at birth. Pediatr Res. 2005;58(6):1295-9.
57. Buonocore G, Perrone S, Longini M, Paffetti P, Vezzosi P, Gatti MG, Bracci R. Non protein bound iron as early predictive marker of neonatal brain damage. Brain. 2003;126(Pt 5):1224-30.
58. Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. 2006;66(2):355-64.
59. Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta. 2009;1790(7):694-701.
60. Juul SE, Zerzan JC, Strandjord TP, Woodrum DE. Zinc protoporphyrin/heme as an indicator of iron status in NICU patients. J Pediatr. 2003;142(3):273-8.
61. Wallach I, Zhang J, Hartmann A, van Landeghem FK, Ivanova A, Klar M, Dame C. Erythropoietin-receptor gene regulation in neuronal cells. Pediatr Res. 2009;65(6):619-24.
62. Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60(4):386-92.
63. Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138(6):1107-18.
64. Kumral A, Tugyan K, Gonenc S, Genc K, Genc S, Sonmez U, Yilmaz O, Duman N, Uysal N, Ozkan H. Protective effects of erythropoietin against ethanol-induced apoptotic neurodegenaration and oxidative stress in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2005;160(2):146-56.
65. Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59(4):419-25.
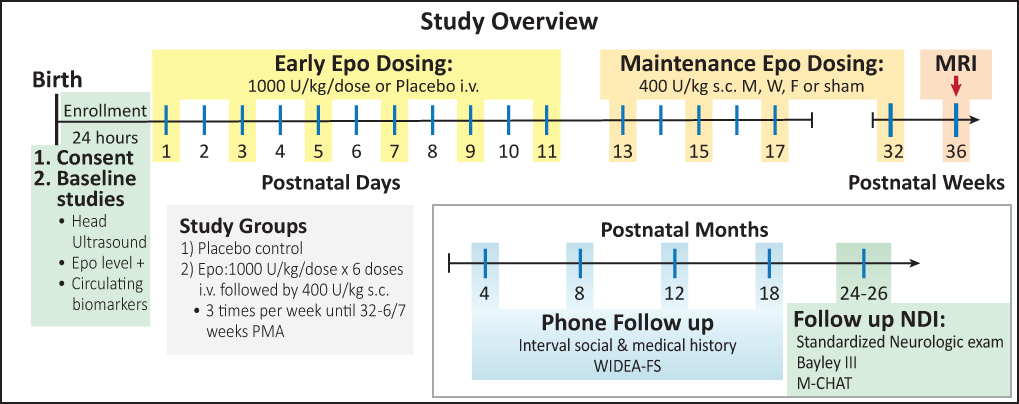
940 babies will be enrolled at 16 centers across the US. Half of the babies enrolled in the study will get standard care plus Epo, and half will get standard care plus a placebo (an inactive substance with no medicine). Babies will be followed every 4 months by phone contact until they are 2 years old, and tested in person at 2 years of age to see how they are developing.
Epo is commonly used in preterm infants, children and adults to help them make red blood cells (these are cells that carry oxygen in the blood). The dose used for making red blood cells has been well tested and is safe in premature babies, but the dose needed for brain protection is higher. Epo is considered experimental when given to premature babies to try to protect the baby from neurological (brain) damage. We are doing this research to find out if the research doses are safe in babies and to test whether babies who receive this drug have better outcomes. Read more About Epo.
Babies entering the study will have a head ultrasound done before receiving any study drug. Half the babies will receive Epo and half will receive the inactive medicine. The first 6 doses of study medication will be by intravenous catheter (IV), and will be given every other day starting on day one. The placebo dosing procedure is identical to Epo dosing for the first 6 doses, but no active medicine will be given. The later doses of Epo will be given by subcutaneous injection (shot), which is the recommended way of giving this medication. If your child is in the placebo group, no further doses or shots will be given.
We will measure the Epo levels in the baby's blood at 4 specific times before and after treatment. We will also test for the effects of Epo at these times. The total amount of blood needed for this study is less than 1/2 teaspoon and will not change the need for a transfusion.
We will collect information from the mom and baby's medical records. The information collected will include information about the pregnancy, the baby's gestational age, weight, body length, blood pressure, lab tests, head measurements, and medical problems that occur during their NICU stay. We will do this until the child is discharged from the hospital. If infants are transferred to another hospital before going home, we will continue to follow them until they go home. We will follow the babies development until they are 2 years old.
Epo is often used in preterm infants to help them make red blood cells. Epo treatment is effective when used this way; however, the dose of Epo that we will use in this study to test for brain protection is higher and there may be unknown and unexpected risks for infants. High doses of Epo have been studied in newborns and no safety concerns were noted. Epo will increase the production of red blood cells, and may increase the blood pressure. We will carefully monitor your infant for any side effects that Epo may have.
The risks associated with erythropoietin (Epo) include:
Read more about the trial on the clinicaltrials.gov website.
Sixteen sites across the country are enrolling eligible infants into the PENUT trial. For more information about each site please click on the link below to be added:
Bailey M Clopp
PENUT Trial Coordinator
cloppb@uw.edu
425-359-1853